Johnson & Johnson forced to pay $9.9 million over role in vaginal mesh scandal
This is good news for campaigners...
NICE sparked outrage when it ruled that the NHS could resume its use of vaginal mesh earlier this month, but over in the US, campaigners are celebrating a big win.
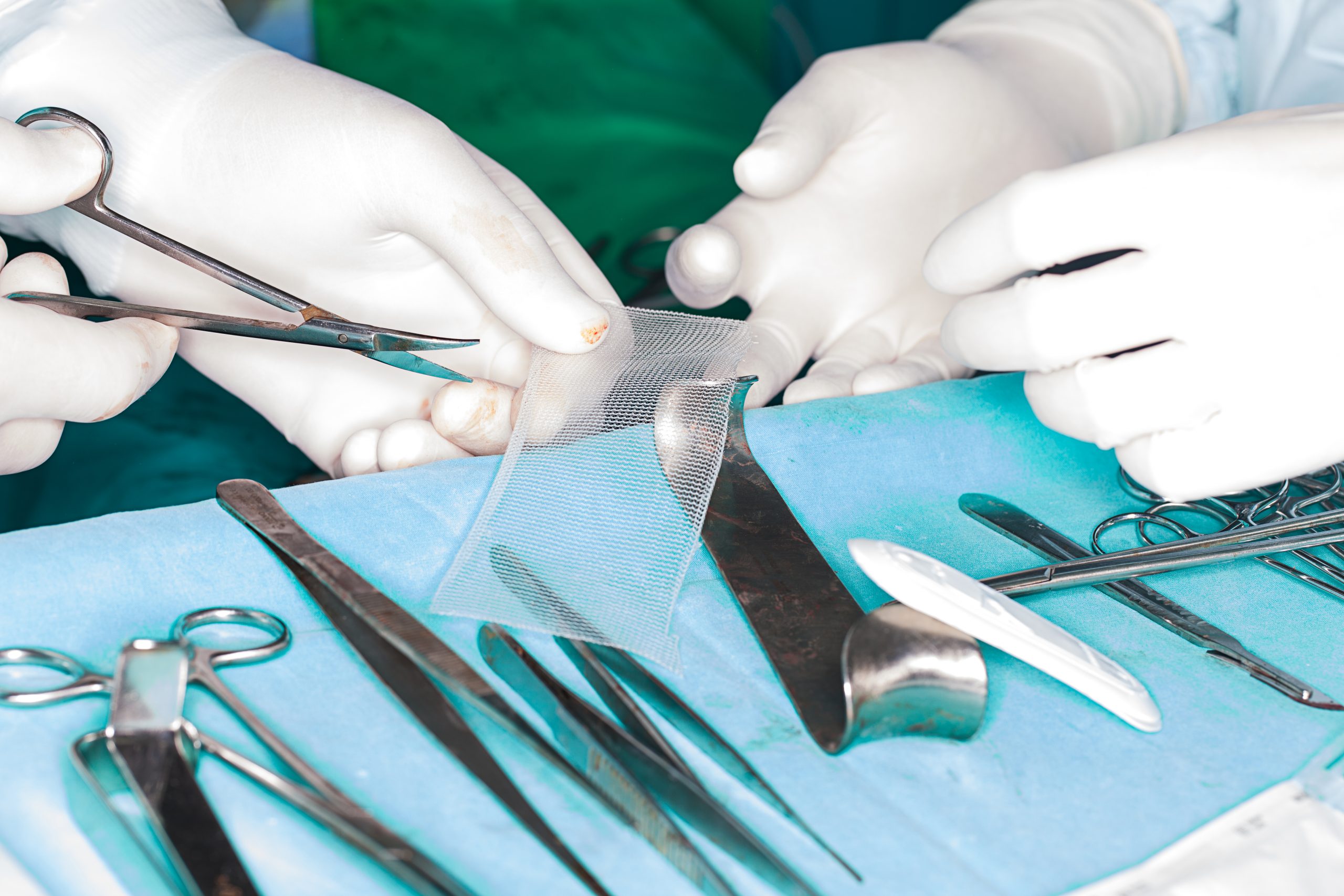
Women who have had the devices, which are made of polypropylene mesh, implanted to treat pelvic organ prolapse or urinary issues like incontinence have reported complications including chronic pain and infections, and even permanent nerve damage.
MORE:Vaginal Mesh Implants: The biggest medical scandal of our generation
Johnson & Johnson, who made the surgical mesh devices, were due to stand trial in Washington this week, after Attorney General Bob Ferguson accused them of misrepresentation and failure to disclose serious risks in their instructions and marketing materials.
But rather than go to trial, the medical giant opted to settle out of court, paying out a whopping $9.9 million.
In a deposition, the company’s Global Head of Medical Affairs, Piet Hinoul, admitted that Johnson & Johnson knew about serious risks including chronic pain and pain with sexual intercourse ‘from day one’, but didn’t disclose them to patients.
Sign up to our free daily email for the latest royal and entertainment news, interesting opinion, expert advice on styling and beauty trends, and no-nonsense guides to the health and wellness questions you want answered.
Ferguson announced that the payment would go towards helping women who’d had pelvic mesh implants. The Attorney General’s Office estimates that hundreds of Washington women have suffered life-altering complications from vaginal mesh surgery.
One woman reported that she was housebound and suffering from depression and loneliness as a result of chronic urinary tract infections, leg cramps and back pain.
Others flagged up how tough it was to remove the mesh in the event of complications, with one woman going through three rounds of surgery for pain and incontinence to remove just part of the device. Another woman revealed that her doctor had told her that trying to remove the mesh was like ‘trying to remove chewing gum from hair’.
In some cases, the consequences are irreversible. One doctor told Johnson & Johnson that their patient’s vagina was ‘permanently destroyed’ due to vaginal mesh in 2009.
"Johnson & Johnson’s knowing deception caused women to suffer in deeply personal ways," Ferguson declared. "I’m proud of my team for holding a powerful interest accountable for its egregious conduct."
Kath Sansom, of Sling the Mesh, who campaign on behalf of the women affected by pelvis mesh implants, has recently spoken out about more action needs to be taken against the scandal - and fast.
"The FDA was so concerned for women's safety that it banned vaginal prolapse mesh. The move came just two weeks after UK health watchdog NICE unbanned this type of mesh, having banned it 18 months earlier in December 2017," she told woman&home. "The move makes a mockery of NICE guidelines. If we cannot trust them to get it right on vaginal prolapse mesh how can we trust them to get it right on guidelines for incontinence mesh slings and abdominal prolapse mesh."
"All women's pelvic mesh has the potential to cause serious, life changing, irreversible damage to women. The longer the time goes on the more women will start coming forward suffering as mesh has the potential to sit like a ticking time bomb for years. Sling The Mesh demands a 20 year retrospective audit to work out the true scale of this horrendous women's health scandal."
Samantha Simmonds is a freelance journalist, content writer, copywriter, and editor based in London.
She graduated from Reading University with a First Class degree in Psychology, later achieving a Distinction in her Diploma in Fashion & Personal Styling from The London College of Style.
Samantha is currently creating digital editorial content for John Lewis, writing for The Edit's wellbeing channel. She also writes for publications including Women's Health, Top Santé, Refinery29, GoodtoKnow, Cosmopolitan, Healthy, Health & Wellbeing, woman&home, and Yahoo, and has created commercial content for brands including Berghaus, Amazon, and Regaine.